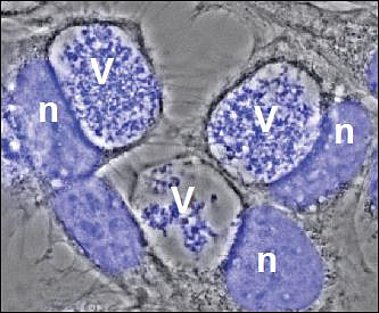
V= Vacuole filled with bacteria
n= host cell nucleus
After uptake into mammalian cells by microfilament-dependent endocytosis, C. burnetii remains in a membrane-bound vacuole. This C. burnetii-containing vacuole (CCV) initially appears to mature similarly to phagosomes containing avirulent bacteria, undergoing fusion with endosomes and lysosomes, resulting in the formation of a phagolysosomal compartment. However, C. burnetii delays the maturation of the CCV probably through interactions between the early CCV and the autophagic pathway. How C. burnetii mediates establishment of the phagolysosomal-like compartment in which it resides and replicates, is not well understood. However, a functional type IV secretion system (T4SS) is required, suggesting that bacterial effector proteins may directly influence biogenesis of the C. burnetii-occupied vacuole.
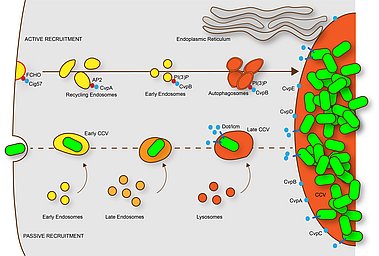
This scheme describes the maturation of C. burnetii-containing phagosomes (CCV). Within minutes after uptake into macrophages, the CCV fuses with early endosomes. Later the CCV fuses with late endosomes and finally with lysosomes. However, the phagosome maturation is delayed, possibly by interaction with the autophagic pathway. The resulting phagolysosome contains hydrolases and has a low pH (4.5-5.5), which is required for the activation of the Dot/Icm T4SS and the translocation of effector proteins. C. burnetii vacuolar proteins (CvpA-E) localize at CCVs and participate in its biogenesis. CvpA binds AP2 on recycling endosomes re-orienting the trafficking of these compartments to the CCV; CvpB binding to PI(3)P at early endosomal membranes inhibits the activity of the PI kinase PIKfyve, thus promoting autophagy and CCV homotypic fusion. The effector protein Cig57 binds FCHO at clathrin-coated vesicles, re-routing their trafficking to CCVs. C. burnetii also actively induced the formation of contact sites between the ER and the CCV. From Lührmann et al., 2017.
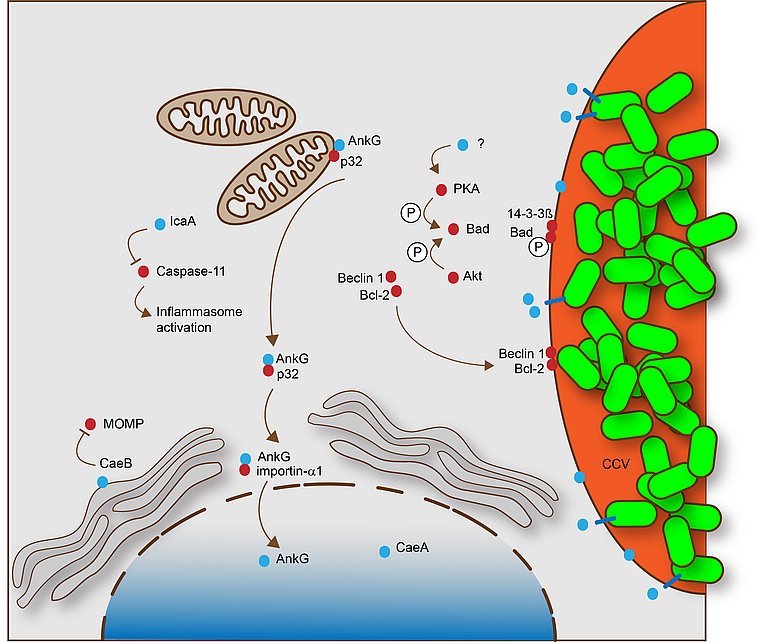
This scheme describes the mechanisms by which C. burnetii inhibit apoptosis and pyroptosis. These processes are dependent on a functional Dot/Icm T4SS. The T4SS effector proteins that specifically participate in apoptosis or pyroptosis inhibition are shown in blue, while host cell proteins involved in this process are shown in red. From Lührmann et al., 2017.
C. burnetii has been shown to inhibit host cell apoptosis. Apoptosis is a programmed cell death pathway that is crucial for immune system maintenance and removal of damaged or infected cells. It was demonstrated that C. burnetii infection inhibits the induction of the intrinsic cell death pathway by preventing cytochrome C release from mitochondria and consequently inhibiting caspase 3 activation. Another study showed that C. burnetii infection inhibited not only the intrinsic but also the extrinsic apoptosis pathway. While the mechanism(s) of C. burnetii-induced inhibition of host cell apoptosis is not well understood, it is clear that it depends on a functional T4SS.
Our current projects
1.) Analysis of the T4SS effector-induced inhibition of apoptosis
To determine the diverse mechanisms employed by Coxiella burnetii to prevent host cell apoptosis, we will analyze the function of Coxiella burnetii T4SS substrates that interfere with signaling through the intrinsic and extrinsic apoptotic pathways.
2.) Host and Pathogen Determinants of Resolution or Chronic Inflammation in Coxiella burnetii Infection
We aim to identify how host and pathogen determinants influence cell death, cytokine secretion, and intracellular signalling in Coxiella burnetii infection. Thus, by investigating both host and pathogen factors we aim to clarify how Coxiella burnetii infection resolves or develops into chronic inflammation. (For more information please see: https://www.sfb1181.forschung.fau.eu/ )
3.) Q-GAPS – Q fever GermAn Interdisciplinary Programm for reSearch subproject 7
C. burnetii infects a variety of host species. The infection route as well as the disease severity seems to be species-specific. To gain insight into the reasons underlying these differences we will investigate the interaction of C. burnetii with cells form different host species. (For more information please see: https://www.q-gaps.de).
Contact: info(at)q-gaps.de





