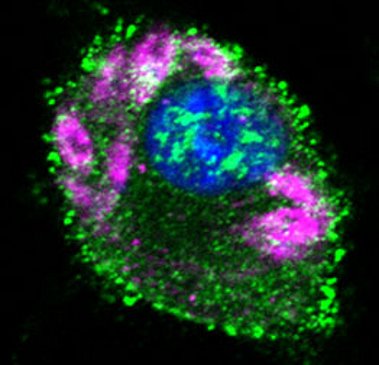
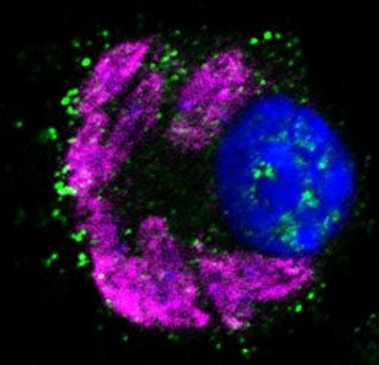
(images: courtesy to Katrin Paduch, Institute of Clinical Microbiology, Immunology and Hygiene, FAU)
Research Topics of the Laboratory for Innate Immunity and Leishmania Research
Leishmania parasites are protozoan pathogens which are transmitted by sand flies to various mammalian hosts, where they cause a broad range of diseases ranging from local and ultimately self-healing lesions to systemic, visceral infections that are lethal, if untreated. Studies with well-established mouse models of leishmaniasis revealed that both the innate and adaptive immune system (macrophages, dendritic cells (DCs), natural killer (NK) cells, CD4+ T helper (Th) cells, CD8+ T cells, B lymphocytes and different cytokines such as IFN-g, TNF and type I inferferons) contribute to an efficient immune response against the intracellular parasites.
The focus of our research work is the analysis of cells, receptors, cytokines and antimicrobial effector mechanisms of the innate immune system during Leishmania infections. Using high resolution imaging techniques, genetic approaches, immunophysical methods and mouse models we aim to elucidate the immunological processes and the tissue micromilieu that account for the control or evasion of the intracellular protozoan parasites Leishmania major, Leishmania tropica, Leishmania infantum, Leishmania mexicana and Leishmania braziliensis and to define novel approaches for the treatment of human leishmaniasis.
In current projects special emphasis is given to the
A) function and regulation of arginase 1, arginase 2 and inducible nitric oxide synthase (iNOS, NOS2) during cutaneous leishmaniasis (L. major and L. mexicana) (supported by the German Research Foundation, SFB 1181 “Checkpoints for resolution of inflammation”, project C4)
B) epigenetic regulation of arginase 1 by tumor necrosis factor (TNF) and interferon-g in visceral leishmaniasis (L. infantum) (Infect-EraNet, EU/BMBF)
C) mechanisms of TNF-mediated control of intracellular pathogens in mice and man (supported by the Interdisciplinary Center for Clinical Research, University Hospital of Erlangen, project A63)
D) crossregulation of inducible nitric oxide synthase (iNOS, NOS2) and iron in cutaneous and visceral leishmaniasis
E) Toll-like receptor 9-dependent regulation of the immune response in visceral leishmaniasis (L. infantum) (supported by the German Research Foundation, Research Training Group 1660, project A5)
F) role of natural killer cells and innate lymphoid cells (ILC1, ILC2) during cutaneous (L. major) and visceral (L. infantum) infection (supported by the German Research Foundation, SPP 1937 “Innate lymphoid cells”)
G) therapeutic and immunoregulatory effects of reactive chlorine oxygen species in human cutaneous leishmaniasis (together with Waisenmedizin e.V. Freiburg https://waisenmedizin.org/ )
Recent key publications
- Messlinger H, Sebald H, Heger L, Dudziak D, Bogdan C and Schleicher U. (2018). Monocyte-derived signals activate human natural killer cells in response to Leishmania parasites. Frontiers in Immunology epublished January 24, 2018: doi: 10.3389/fimmu.2018.00024.
- Schleicher U, Liese J, Justies N, Mischke T, Haeberlein S, Sebald H, Kalinke U, Weiss S and Bogdan C. (2018). Type I interferon signaling is required for CpG-oligodesoxy-nucleotide-induced control of Leishmania major, but not for spontaneous cure of subcutaneous cure of primary and secondary L. major infection. Frontiers in Immunology epublished January 11, 2018: doi: 10.3389/ fimmu.2018.00079.
- Schleicher U., Paduch K., Debus A., Obermeyer S., König T., Kling J.C., Ribechini E., Dudziak D., Mougiakakos D., Murray P.J., Ostuni R., Körner H. and Bogdan C (2016). TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Reports 15: 1062-75.
- Bogdan C (2015) Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol 36: 161-178
- Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, Fang FC, Bogdan C, Weiss G (2013). Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med. 210: 855-873.
- Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, Bogdan C (2007). NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of pDCs. J Exp Med. 204: 893-906.





