Molecular biology of Leishmania virulence
Leishmaniasis is a worldwide prevalent infectious disease. This disease is primarily encountered in tropical and subtropical countries, with an estimated number of 0.6 to 1 million new cases of cutaneous leishmaniasis and 50,000 to 90,000 new cases of visceral leishmaniasis leading to 20 to 30 thousand deaths per year. Our research focuses on Leishmania major parasite that is the causative agent of cutaneous leishmaniasis, a disease transmitted to human by sand fly bite. In its insect vector, the parasite resides in a highly infective extracellular promastigote form. Once in its mammal host, the parasite enters myeloid host cells with a high prevalence for macrophages. There, they transform into intracellular amastigotes, which facilitate the spread in the host organism. To survive during the infection of mammalian hosts, Leishmania parasites must quickly adapt to their new and hostile environment. To this aim, parasites produce a range of virulence factor that will interfere with the pro-inflammatory signaling pathways that should normally be triggered by the infection in order to rend the macrophages unresponsive (figure 1) (Soulat & Bogdan, Front Immunol. 2017). Overcoming this virulence strategy could protect against Leishmania infection. However, only few Leishmania virulence factors have been characterized to date.
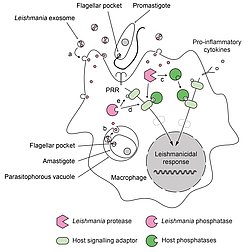
Figure 1: Role of phosphatases in the deactivation of host cell during Leishmania infection.
Leishmania parasites have developed a dual strategy to inactivate important signaling pathways in their host cell. During infection, promastigotes and amastigotes secrete exosomes containing virulence factors. These exosomes release their content into the infected cells after fusing with (a) cytoplasmic membranes (in the case of extracellular promastigotes) or (b) phagosomal membranes (in the case of intracellular amastigotes). Secreted Leishmania proteases can proteolytically activate host phosphatases (c) that subsequently down regulate activating cellular pathways. Secreted Leishmania phosphatases could synergize with host phosphatases (d) or act independently (e) to modulate the infected cell response.
Characterization of new virulence factors
1- Our laboratory has recently identified a new virulence factor produced by Leishmania parasites named LmPRL-1 (Leitherer, Infect Immun. 2017). The characterization of LmPRL-1 revealed that this phosphatase was preferentially expressed and secreted by promastigotes via the exosome route. This allowed the phosphatase to reach the cytosol of infected macrophage. If we could demonstrate that LmPRL-1 expression promotes Leishmania virulence, we are still investigating in vivo and in vitro the biological mechanisms supporting LmPRL-1 functions.
2- We are also currently investigating an homologue of LmPRL-1 that is preferentially expressed by the intracellular amastigote form of Leishmania. This phosphatase that we named LmPRL-2 has been biochemically characterized and, like for LmPRL-1, deletion mutant were developed using the CRISPR technics to study its involvement in the parasite virulence.
Both projects aim to characterize new virulence factors of Leishmania and use a large range of technical approaches to understand how LmPRL phosphatases can alter the host-pathogen interaction. A better understanding of the Leishmania virulence will open new avenues to develop therapeutic strategies.





